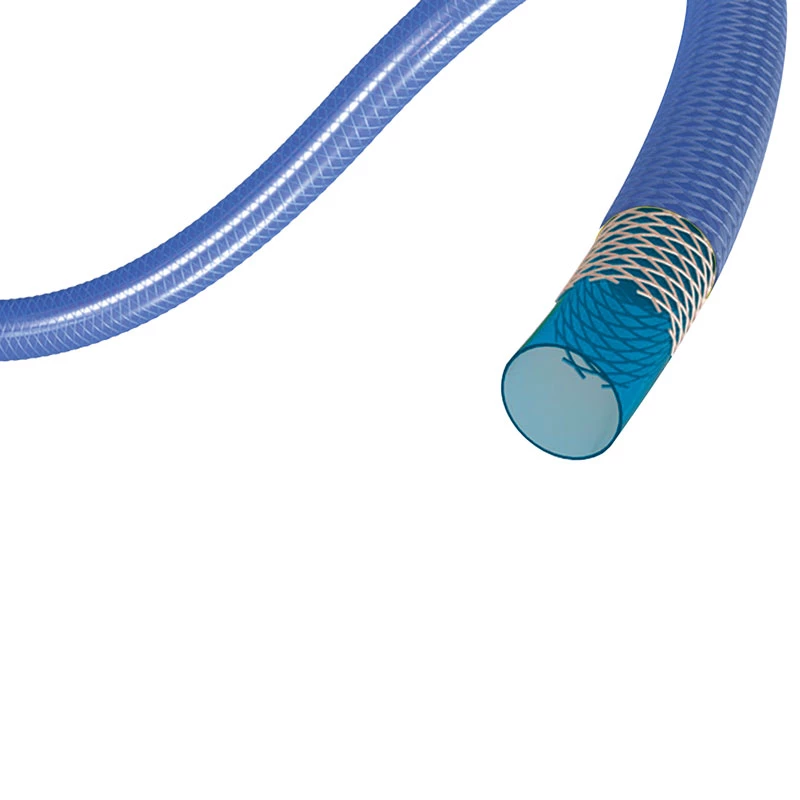
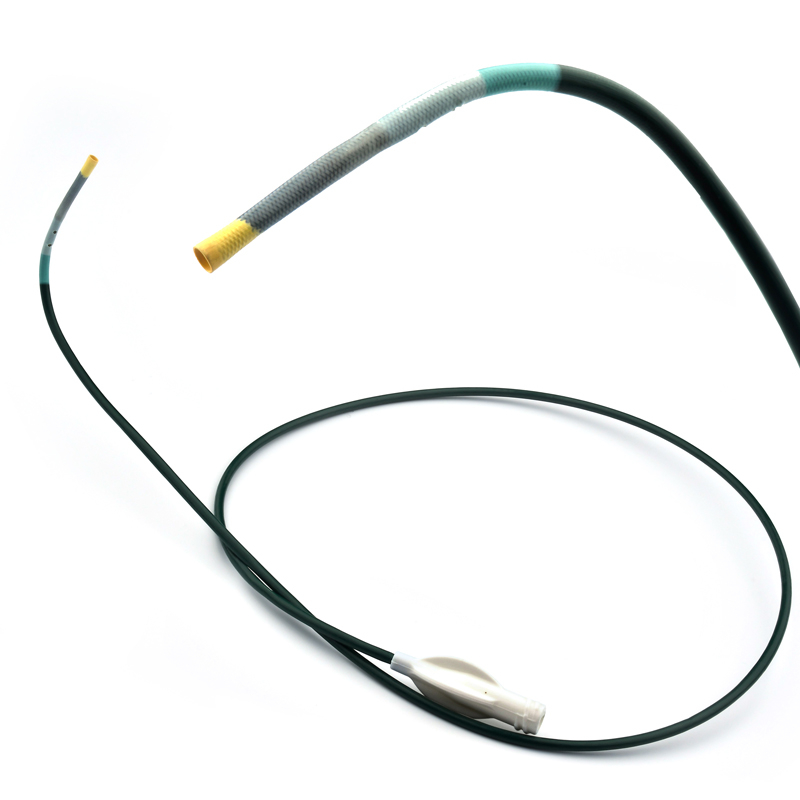
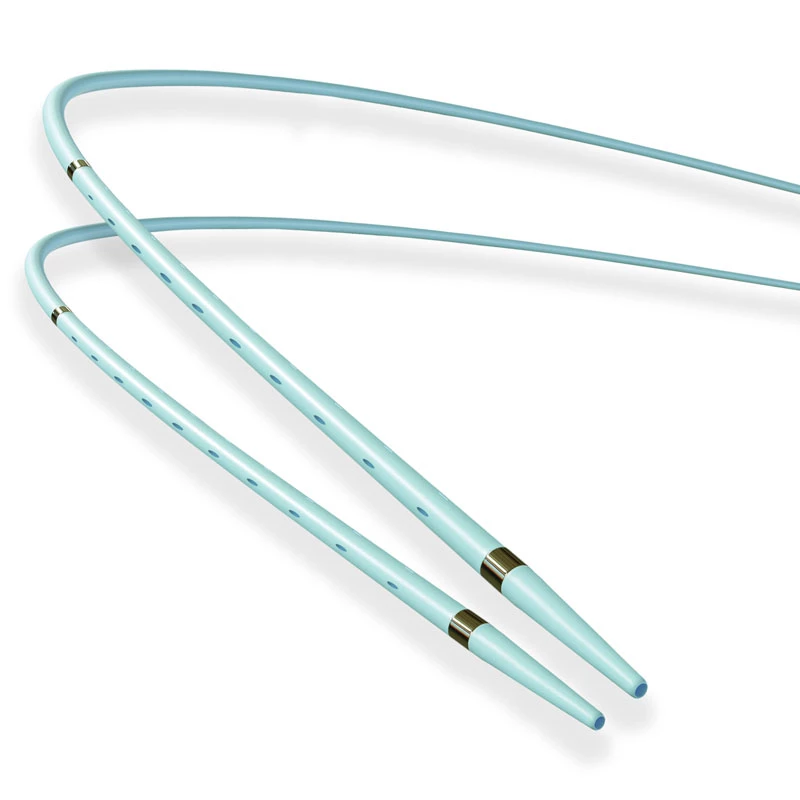
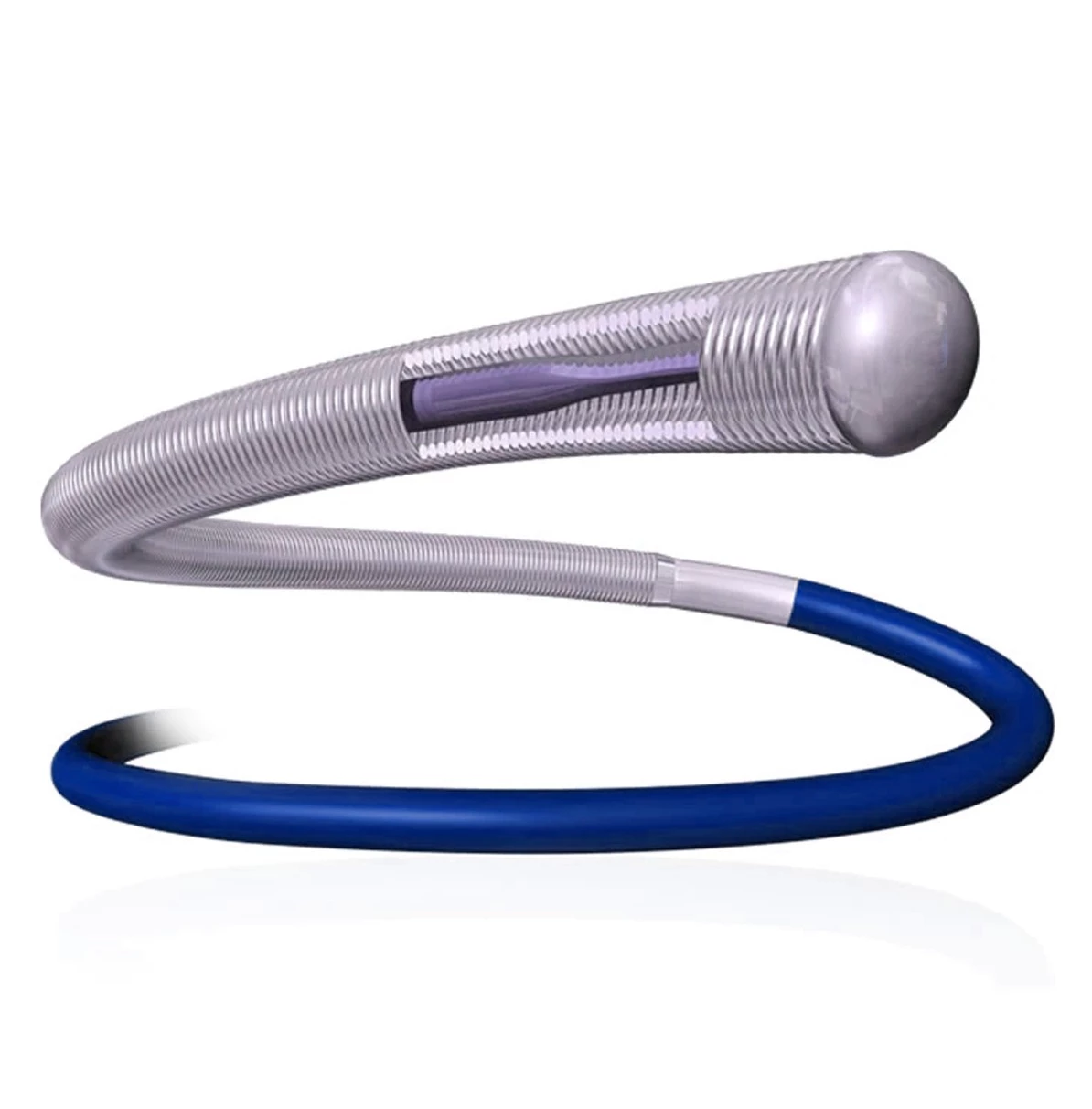
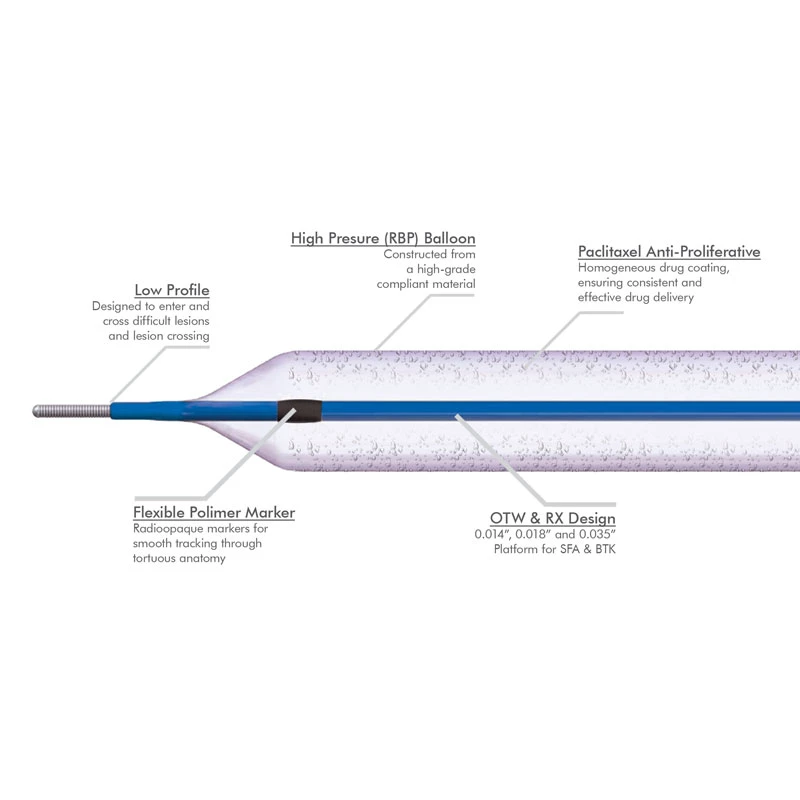
Extender
PTCA Drug Eluting Coronary Balloon
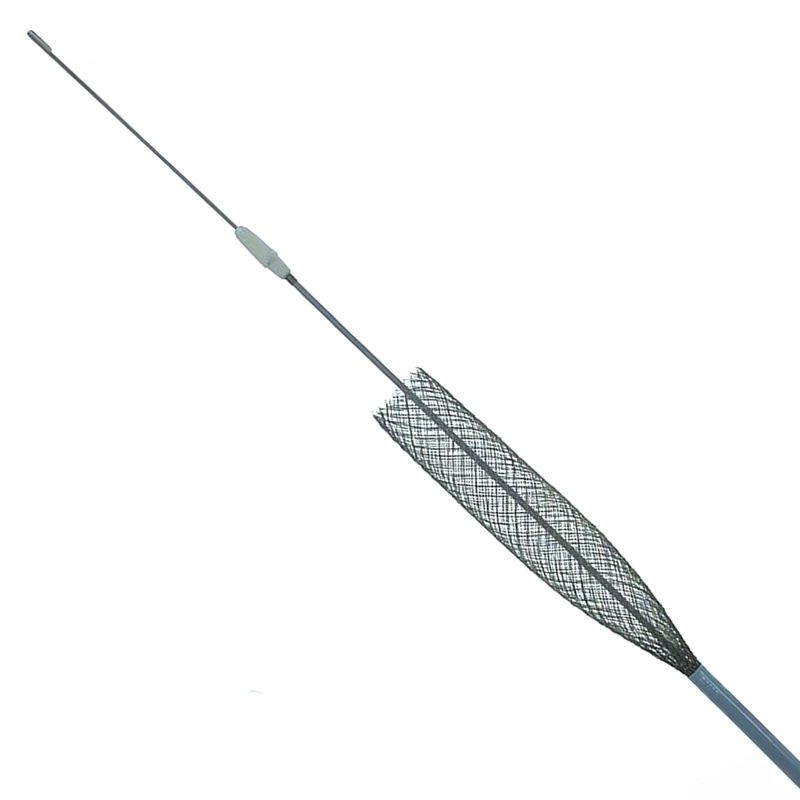
The extender is a drug-eluting balloon dilatation catheter designed for percutaneous transluminal coronary angioplasty (PTCA) and has been optimized for the treatment of patients with coronary arterial disease.
| Balloon Diameter | 2.0 mm - 5.0 mm | |
| Balloon Lenght | 10 mm, 15 mm, 17 mm, 20mm, 26 mm, 30mm | |
| Catheter Shaft | RX | |
| Catheter Lenght | 120 cm,150 cm | |
| Radiopasity | Pt-Ir Marker | |
| Applicable Guidewire | 0.014" | |
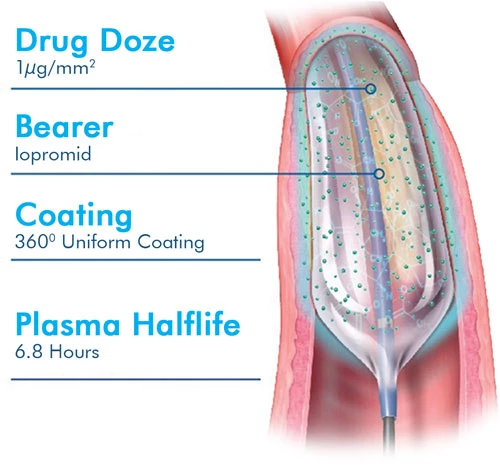
| Drug/Quantity | Paclitaxel 3.0 - 3.5 μg/mm2 |





Detailed specifications
ExtenderⓇA Drug-Eluting PTCA Balloon Catheter is used to treat coronary vessels with a balloon structure that releases drugs at the tip.
It treats interventional treatment of coronary artery disease by providing fast and effective drug delivery.
HIGH PERFORMANCE LONG-TERM EFFECTIVE CLINICAL RESULTS
Mechanism of Action
Arterial periphery injuries provoke an inflammatory reaction with the excretion of growth factors that trigger the onset of cell division and smooth November cell migration by balloon dilatation.
Indications
Myocardial perfusion or coronary artery bypass stenosis or occlusion to provide or balloon dilatation of the endoprosthesis, including with thin veins after treatment with the remaining stenosis and coronary dilatation of endovascular prostheses of pre-and post-dilatation indicated.
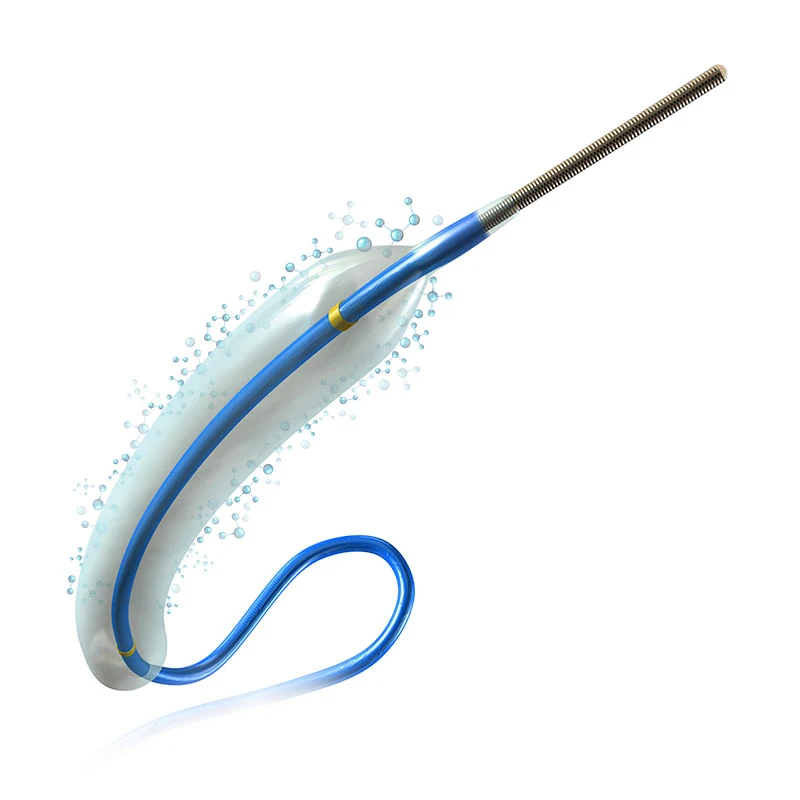
ExtenderⓇPTCA catheter is indicated for the dilatation of the affected segments of a coronary artery or a coronary bypass to enhance myocardial perfusion. This paclitaxel-eluting balloon feature a proper coating technology that consistently delivers paclitaxel, an anti-restenotic drug during very brief inflation times, while also minimizing washout of the drug during delivery and placement of the drug-eluting balloon. Balloon catheters offer excellent pushability, trackability, and crossability due to a low balloon profile, low tip entry profile, and hydrophilic coating on the distal shaft of the catheter. Paclitaxel eluting coronary balloon catheter is especially indicated for the treatment of coronary in-stent restenosis.
Major inclusion criteria
- Signed data release form
- Patients with the restenotic lesion in a previously stented area of a coronary artery (irrelevant whether BMS or DES related)
- Target reference vessel diameter: 2 - 4.5 mm
- Target lesion length: 8 - 28 mm
- Target lesion stenosis: > 50 % - < 100 %
- Patient with allergy against appropriate anticoagulation/antiplatelet therapy
Patients with allergy against paclitaxel Patients with a target lesion that was previously treated by brachytherapy
ADVANTAGES
- Excellent pushability
- Targeted drug delivery into the vascular wall
- Single shot, short-term Paclitaxel delivery for long-term vessel patency
- Homogeneous and complete drug release
- Low profile tip and balloon design for reduced friction and advanced crossing performance
- Homogeneous drug delivery Effectively inhibiting proliferation
- No airborne particles at any time and no premature release of Paclitaxel Hydrophilic coating delivers
- Paclitaxel is homogeneous to the vessel wall instantaneously upon contact
- Hydrophilic coating delivers Paclitaxel without any negative side effects or inflammation
- Load secured to achieve the therapeutic window within 30 seconds of inflation time.
The balloon catheter offers excellent repellency, traceability, and crossability thanks to its low balloon profile, low-end inlet profile, and hydrophilic coating on the distal shaft of the catheter.
Paclitaxel-release coronary balloon catheter is especially indicated for the treatment of intra-coronary stent restenosis.
Paclitaxel prevents restenosis by stabilizing microtubule formation and thus preventing cells from going through replication stages, resulting in inhibition of cell division.
| Dose of Paclitaxel Drug | 3.0 - 3.5 μg / mm2 | |
| Aid Matter | Iopromid | |
| Balloon Diameter | 2.0 mm - 5.0 mm | |
| Catheter Diameter | 3F | |
| Balloon Lenght | 10, 15, 17, 20, 26, 30 mm | |
| Balloon Folding Profile | 2.0 - 4.0 mm: 3 layer | |
| Radiopasity | Pt-Ir Marker | |
| Applicable Guideware | 0.014" | |
| Catheter Shaft | RX | |
| Catheter Lenght | 120 cm,150 cm | |
| Catheter Structure | PA/PEBAX, Hypotube | |
| BALLOON LENGTH (120 cm) | BALLOON LENGTH (150 cm) | |||||||||||
| Diameter | 10 mm | 15 mm | 17 mm | 20 mm | 26 mm | 30 mm | 10 mm | 15 mm | 17 mm | 20 mm | 26 mm | 30 mm |
| (mm) | ||||||||||||
| 2.0 | CR2010M | CR2015M | CR2017M | CR2020M | CR2026M | CR2030M | CR2010X | CR2015X | CR2017X | CR2020X | CR2026X | CR2030X |
| 2.5 | CR2510M | CR2515M | CR2517M | CR2520M | CR2526M | CR2530M | CR2510X | CR2515X | CR2517X | CR2520X | CR2526X | CR2530X |
| 2.75 | CR27510M | CR27515M | CR27517M | CR27520M | CR27526M | CR27530M | CR27510X | CR27515X | CR27517X | CR27520X | CR27526X | CR27530X |
| 3.0 | CR3010M | CR3015M | CR3017M | CR3020M | CR3026M | CR3030M | CR3010X | CR3015X | CR3017X | CR3020X | CR3026X | CR3030X |
| 3.5 | CR3510M | CR3515M | CR3517M | CR3520M | CR3526M | CR3530M | CR3510X | CR3515X | CR3517X | CR3520X | CR3526X | CR3530X |
| 4.0 | CR4010M | CR4015M | CR4017M | CR4020M | CR4026M | CR4030M | CR4010X | CR4015X | CR4017X | CR4020X | CR4026X | CR4030X |